
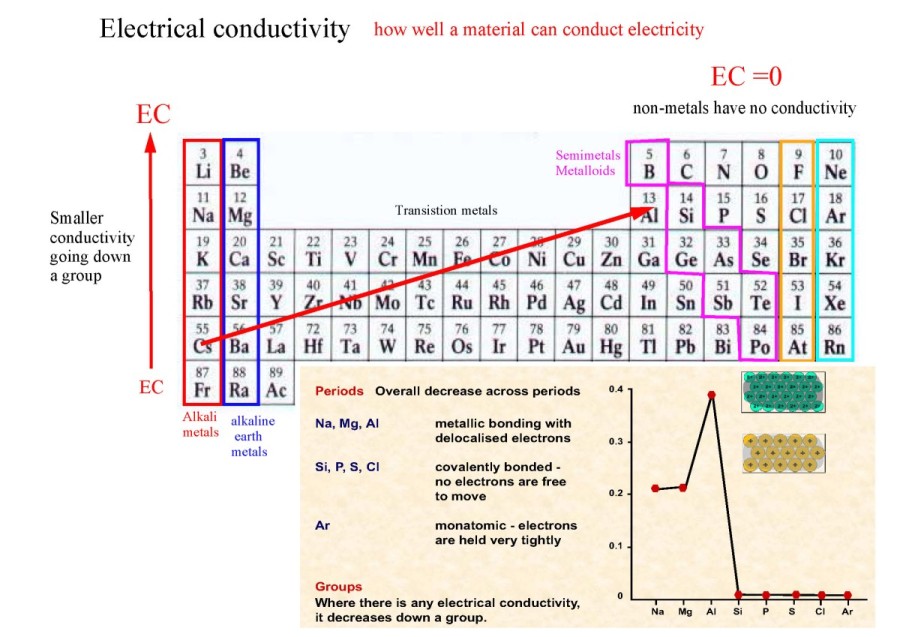
Low = substitution is possible with little or no economic and/or performance impact Medium = substitution is possible but there may be an economic and/or performance impact High = substitution not possible or very difficult. The availability of suitable substitutes for a given commodity. A higher recycling rate may reduce risk to supply. The percentage of a commodity which is recycled. The number of atoms of the element per 1 million atoms of the Earth’s crust. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems.ĭata for this section been provided by the British Geological Survey.Īn integrated supply risk index from 1 (very low risk) to 10 (very high risk). Where more than one isotope exists, the value given is the abundance weighted average.Ītoms of the same element with different numbers of neutrons. This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an atom relative to that of carbon-12. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.ĭensity is the mass of a substance that would fill 1 cm 3 at room temperature. The temperature at which the liquid–gas phase change occurs. The temperature at which the solid–liquid phase change occurs. The arrangements of electrons above the last (closed shell) noble gas. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The atomic number of each element increases by one, reading from left to right.Įlements are organised into blocks by the orbital type in which the outer electrons are found. Members of a group typically have similar properties and electron configurations in their outer shell.Ī horizontal row in the periodic table. We can calculate the effective nuclear charge by subtracting the number of inner shell electrons from the number of protons.A vertical column in the periodic table.
#Na charge periodic table how to
How to Calculate Effective Nuclear Charge Because of the varying charge on electrons in different orbitals, we typically refer to the effective nuclear charge, which is the effect of the nucleus experienced by the outermost electron of the atom, taking into account the shielding effect of inner electrons.
#Na charge periodic table full
The presence of electrons on the inner shells of an atom “shield” the outermost electron from feeling the full positive charge. This results in a varying attraction of the nucleus on the electrons surrounding the nucleus, which is known as nuclear charge. However, negatively charged electrons around the nucleus are organized into layers called orbitals which repel each other, and negate some of the positive charge of the nucleus. Electrons are attracted to the nucleus as they are negatively charged.
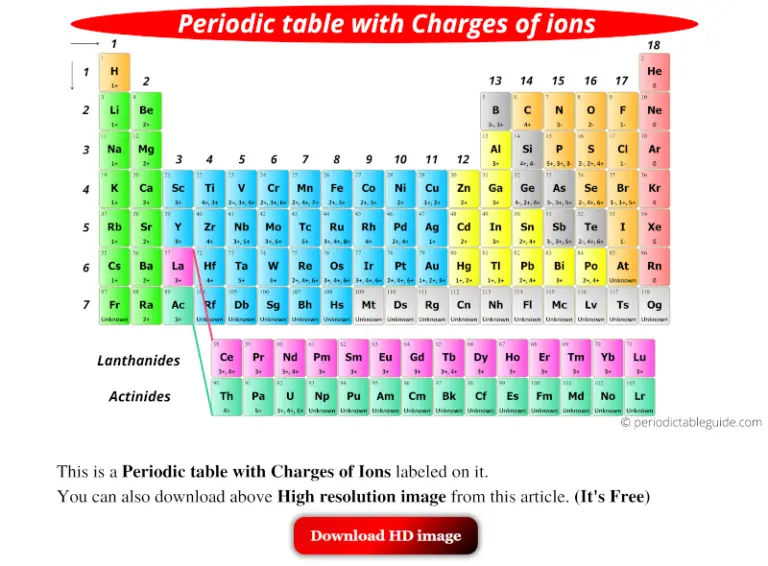
The nucleus of an atom contains positively charged particles called protons. The net attraction on these outer electrons is known as effective nuclear charge. Electrons are attracted to the nucleus as it is positively charged, but electrons in the inner shells can negate some of the attraction of the nucleus on the outermost electrons.
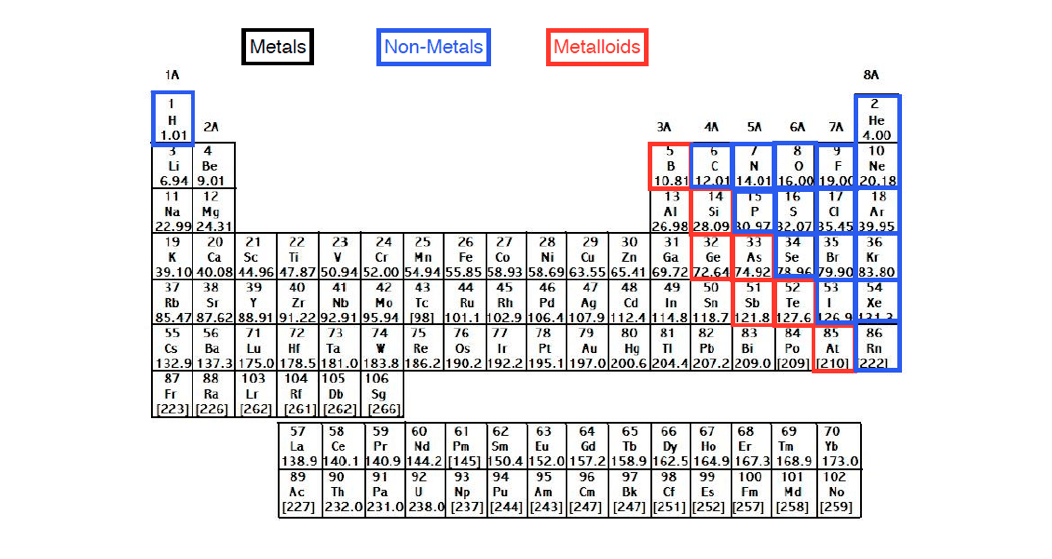
Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. The equation for calculating nuclear charge is Zeff = Z - S, where Zeff is the effective nuclear charge, Z is the number of protons, and S is the number of inner electrons.ģ. You can calculate effective nuclear charge if you know the number of inner electrons and the number of protons of an atom, both which can be found either from the periodic table or from online resources.
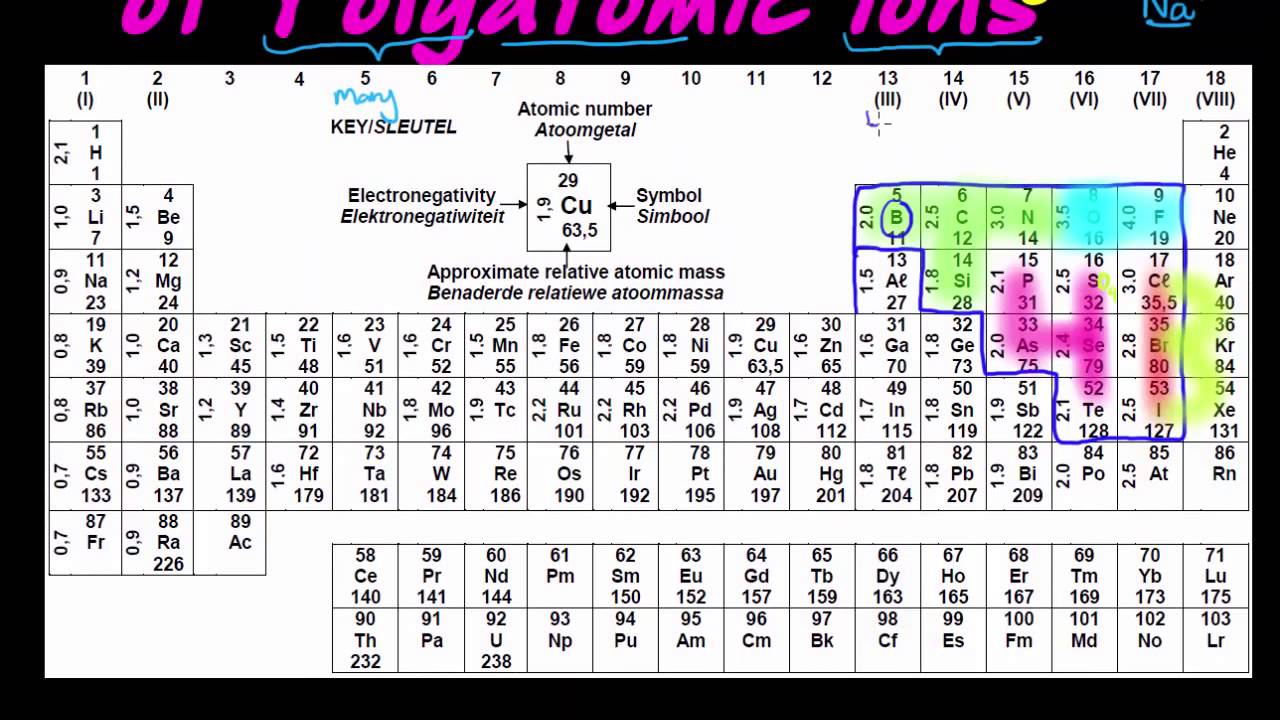
How do you calculate effective nuclear charge? Nuclear charge values have been determined for the elements. These values are recorded in encyclopedias, scientific textbooks, and scientific journal articles.Ģ. How do you find effective nuclear charge? These outer electrons are also known as valence electrons.ġ. Elements in different groups on the periodic table have different numbers of electrons in their outermost shells. These negatively charged electrons are arranged into shells which form layers surrounding the nucleus. Refresher: Atoms are composed of a nucleus, containing positively charged protons and neutral neutrons, surrounded by a cloud of negatively charged electrons.


 0 kommentar(er)
0 kommentar(er)
